3. Thermal physics
Go
3.1 Thermal concepts
##
Temperature and absolute temperature
There have two commonly used measurment of temperature, one is **Celsius(摄氏度)** and the other one is **Kelvin(开氏度)**
The **temperature difference**difference between them is **same**same ${\Delta K}={\Delta C}$
Absolute zero is 0K whcih is -273C273$\Celius$
##
Heat Heat((Internal Energy)
>**Heat**
-
Heat is energy that is
**transferred**transferred from one body to another as a
result of a**difference intemperature**temperature. -
Internal
energy**energy is the**total**total random**kineticenergy**energy of the
particles of a substance,**plus**plus the total inter-particle**potentialenergy**energy of the particles. - The
**heat**heat (temperature) is the**only**only determintion of the**keneticenergy**energy (温度是衡量动能的唯一标准)
>**
>
## specific
Specific heat capacity
Specific heat**heat capacity follow the formula:
$$Q=mc{\Delta T}$$
Q is energy(heat), m is mass, ${\Delta T}$ is Temperature change and c is specific heat capacity.
-
- To define this equation, it means that suppose there's a object A have specific heat capacity c and it required Q energy to raise it's temperature
**1K(C)**- - Different substances have different specific heat capacities because of different physical properties
##
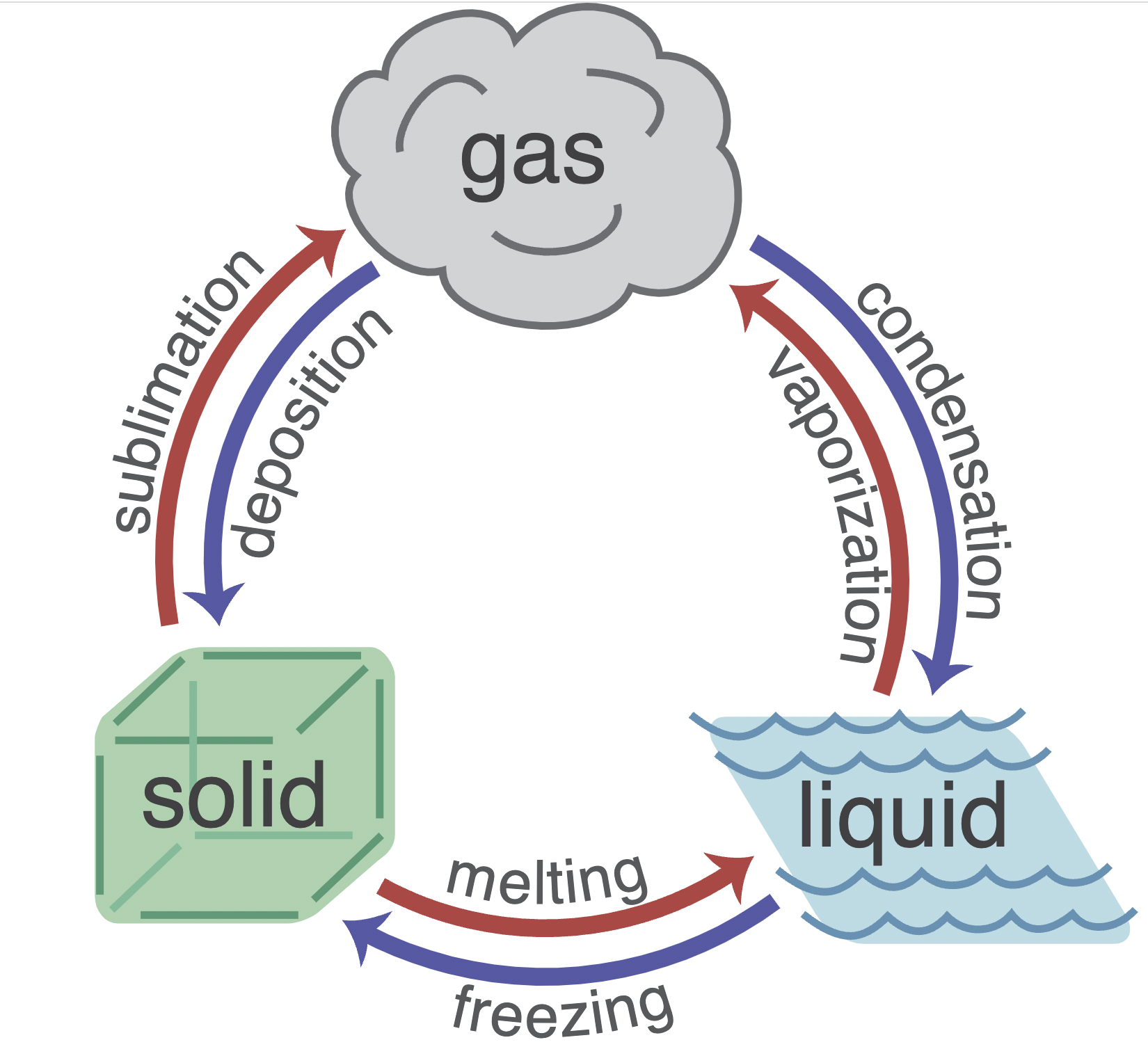
Change of phase
When heating some substances it might not necessarily increase temperature but change phase-
- melting (solid to liquid)
- - freezing (liquid to solid)
- - vaporisation/
boiling(boiling (liquid to gas)- - condensation (gas to liquid)
- - Sublimation(solid to gas)
- - condensation (gas to solid)

Specific latent heat
Specific latent heat follow the formula:
$$Q = mL$$
In this case the L can be replaced by L~V~$L_V$ (vaporisation) and L~F~$L_F$ (fusion)-
- specific latent heat of fusion: The amount of heat required to change
**mkg**mkg of a substance from**solid**solid**toliquid**liquid without any change in temperature.- - Specific latent heat of vaporization: The amount of heat required to change
**mkg**mkg of a substance from**liquid togas**gas without any change in temperature.#
3.2Modelling2 Modelling a gas
##
The Avogadro constant
Avogadro constant $N_A$ is a **experimental constant**constant that measurment the number of particle in one **mole**mole of substance, which $N_A=6.022\times 10^{23}mol^{-1}$
mole is measured by the amnount of particle carried by 12g of Carbon-12 which repersent as $n$
$$n={\frac{N}{N_A}}$$n
Where $n$ is mol number, N$N$ is number of particle and $N_A$ is avogadro constnt##
Pressure
Pressure is defined as the **normal force**force applied **per unit area**area which followed the equation:
$$p={\frac{F}{A}}$$p
Where $p$ is pressure F$F$ is force and A$A$ is area.
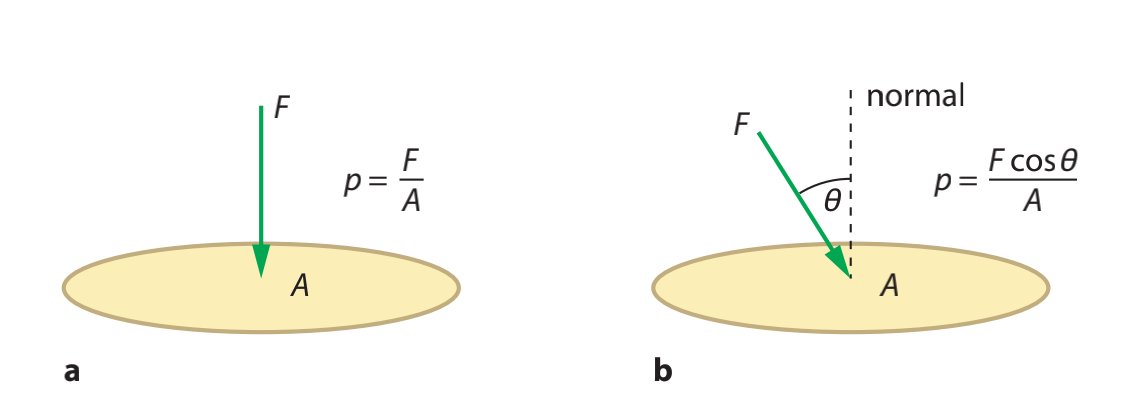
If the force dose **not vertically**vertically acting at an object, then should use the equation:
$$p=\frac{Fcosθ}{A}$$
p in **non-closed**closed condition should be equal to 1atm which is $1.013 × 10^{5}Pa$ on Earth##
Ideal gas
An ideal gas is a theoretical model of a gas which should follow the rules that:-
- The molecules are
**pointparticles**particles, each with**negligiblevolume**volume.- - The molecules obey the laws of mechanics.
- - There are
**noforces**forces between the molecules except when the molecules collide.- - The duration of a collision is
**negligible**negligible compared to the time between collisions.- - The collisions of the molecules with each other and with the container walls are
**elastic**elastic.- - Molecules have a
**range ofspeeds**speeds and**moverandomly**randomly.>
The real gas at high temperature and low pressure will close to ideal gas
{.is-info}
An ideal gas should followed the equation:
$$PV=nRT$$
P is pressure, V is volume, n is mole number, R is ideal gas constant(constant ($8.31Jmol^{-1}K^{-1}$) and T is temperature##
The Boltzmann equation
$$E_{avg}=\frac{3}{2}k_bT$$
This is measurment of average kenetic energy in ideal gas, and $k_b$ means $\frac{R}{N_A}$ which is $1.38\times10^{−23}JK{JK^{−1}$
.
Therefore the average internal energy would bebe:
$$U=\frac{3}{2}pV$$